Bacterial Electrophysiology
Rapid bacterial voltage transients were only recently discovered, raisining numerous questions. What is their physiological function? What are the molecular players that give rise to the transients? Does voltage play a role in pathogensis or antibiotic resistance?
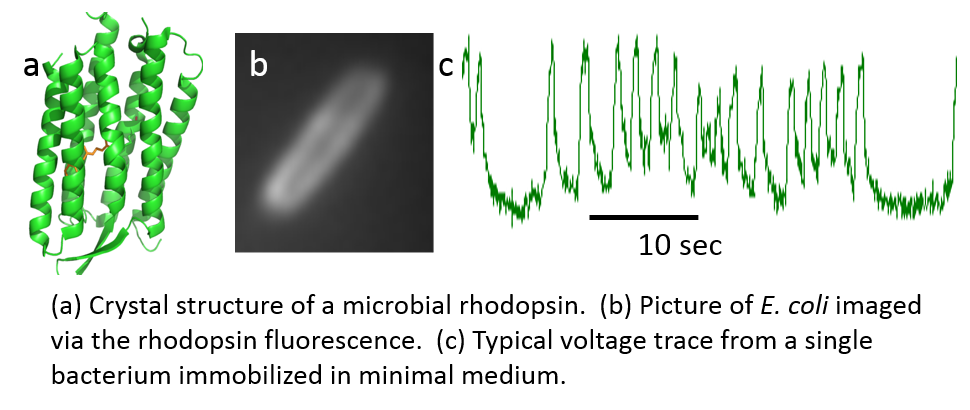
We seek to answer these questions by using microbial rhodopsin voltage indicators to record bacterial electrophysiology.
We revealed E. coli use voltage and calcium to relay a sense of touch, and that mechanical stimulation can induce differences in the protein content of the cell. Our hypothesis is that bacteria can integrate mechanical cues to modulate their lifestyle, similar to chemical signaling. We are currently trying to uncover the molecular basis for bacterial mechanosensation, as well as idnetify chemical modulators which may help uncover downstream effects from voltage and calcium transients.
Yeast pH dynamics
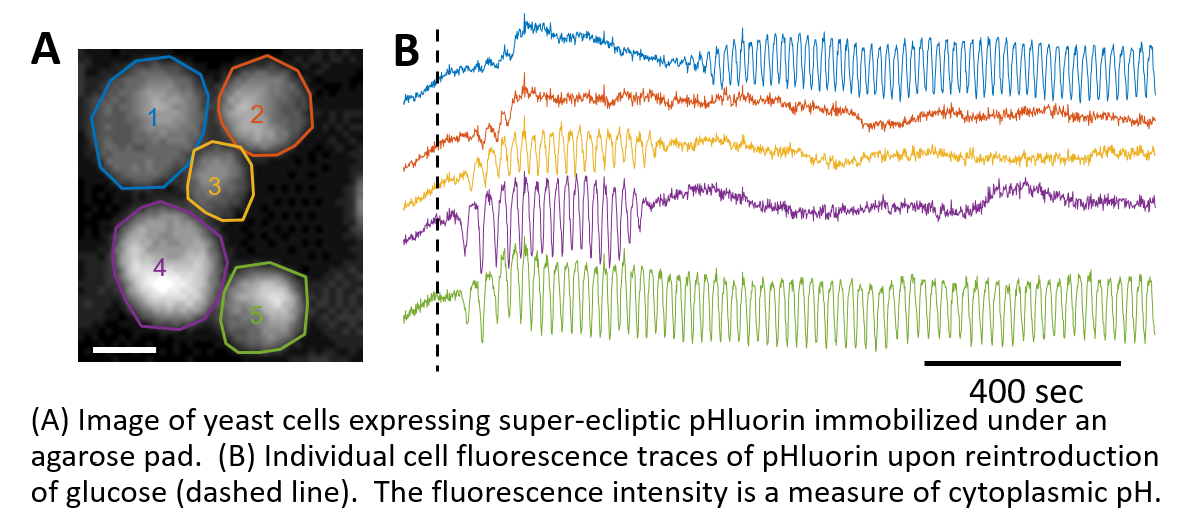
We discovered Saccharomyces cerevesiae change their cytoplasmic pH during glycolytic oscillations (when starved cells are fed glucose). These oscillations occur with a period of 30 seconds, and are concomitant with NADH changes. We find pH oscillations interesting as every reaction in the cytoplasm will be tuned by pH, giving the oscillations the potential to control global reaction rates. We showed that cells undergoing oscillations take longer to bud than non-oscillating cells from the same population, leading us to hypothesize the oscillations could be a means to slow biomass production upon re-energization, enabling other metabolic processes to "boot-up". Cells also oscillated (with a much slower frequency) upon switching from glycolysis to NADPH regeneration.
Automated single cell imaging
Recent advances in microscopy hardware have opened the door to automated single cell imaging, enabling high spatial and temporal resolution data from hundreds to thousands of conditions. We are leveraging these advances, combined with our own image processing and data analysis algorithms, to screen large libraries of mutants and drugs while maintaining single cell resolution. Current projects include screening small molecule libraries to identify modulators of bacterial electrophysiology, and functional genomic screens in E. coli across 4000 ORFs. Our throughput is typically 3, 96 well plates per day, such that the entire Keio collection can be screened, in duplicate, in ~2 months. We are also interested in creating new analytics to perform unbiased data extraction from the rich video data collected.